
Any skin break-in leads to a cascade of biological reactions in the skin whose purpose is to restore its functions as quickly and as well as possible. Skin healing is unfortunately often a complex and unpredictable process, and it is not uncommon to see the development of defective scar processes such as hypertrophic or keloid scars.
Thus, any surgical act or physical aggression on the skin raises the delicate problem of its healing. It is unpredictable and requires the consideration of a risk of developing hypertrophic scars or keloids especially for people at risk.
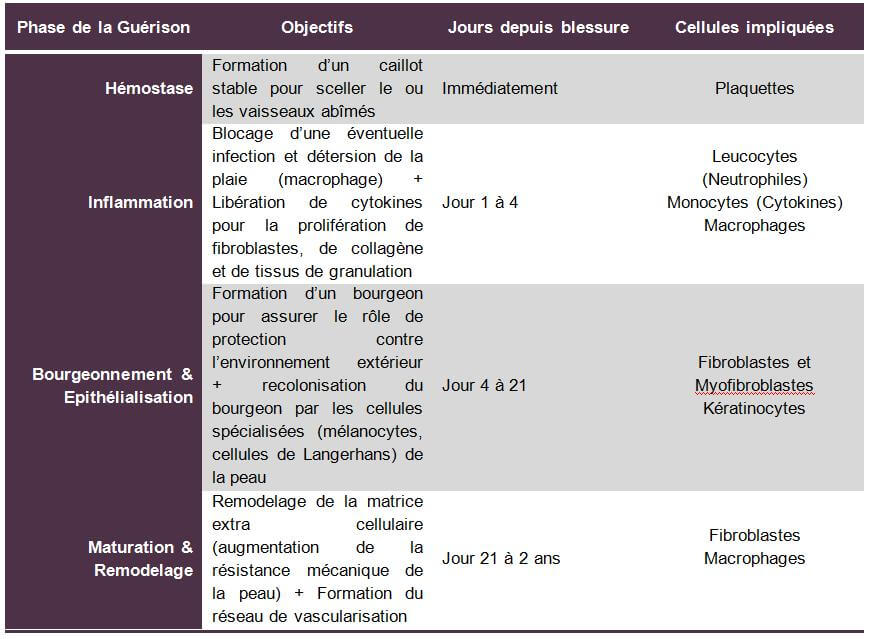
The different phases of healing
The skin is the visible envelope of our body. It plays a vital role, acting as a barrier and exerting a regulatory influence between the external environment and the internal environment. In the presence of an acute or chronic wound, the body triggers a complex healing process whose sole objective is to lead to the closure of the injured skin. We distinguish 4 phases to achieve this healing objective:
The vascular phase or hemostasis
After a physical aggression of the skin, the blood platelet is the cell that seals the damaged blood vessels. They secrete vasoconstrictive substances (pressurization) to stop bleeding. The role of these platelets is to quickly arrive at the formation of a stable clot to seal the damaged vessel.
Adenosine diphosphate (nucleotide) flowing from damaged tissues allows platelets to bind together and cling to exposed collagen. Platelets allow the secretion of thrombin and then fibrins that reinforce platelet aggregation thus producing the stable clot capable of sealing the damaged vessel or vessels.
The vascular phase occurs within minutes of the aggression of the skin.
The inflammatory phase
Following the vascular phase and once the stable clot playing a role of temporary extracellular matrix is formed, the inflammatory phase begins, which lasts from a few hours to a few days depending on the severity of the wound.
This phase begins with a phenomenon of vasodilation (depressurization) thus allowing the circulating cells to reach the wound. Leukocytes thus quickly reach the wound and secrete enzymes that promote the penetration of specialized cells into the wound. Then, thanks to the action of leukocytes promoting cell penetration at the wound level, monocytes attach to the endothelial cells and migrate into the wound. Once in the tissue medium, they differentiate into macrophages and adhere to the proteins of the extracellular matrix. Macrophages play an anti-infectious role and local detersion thanks to their phagocytosis capabilities, they also participate in matrix remodeling. These same monocytes then play a key role in releasing cytokines (Insulin Grow Factor 1, Transforming Grow Factor and Tumor Necrosis Factor). These substances have the property of amplifying the inflammatory response and stimulate the proliferation of fibroblasts, collagen and granulation tissues. Within 72 hours of the appearance of the wound, macrophages become predominant in number and activity. Around the 7th day, few inflammatory cells are identified in the wound and fibroblasts become the predominant cell type.
The budding and epithelialization phase
After the elimination of tissue waste by macrophages and the establishment of a temporary extracellular matrix (stable clot), the reconstitution of a new definitive cell matrix will take place thanks to budding. This phase begins 4 days after the onset of the wound and can last up to 21 days for large wounds. The scar has the shape of a red and rough tissue to the touch. Fibroblasts will play the role of backbone to this matrix by secreting collagen (type I and III) which acts as a framework on which the regenerated cells will be grafted. Endothelial cells (keratinocytes) and fibroblasts will proliferate in this way until new blood vessels are formed and a new extracellular matrix is in place. Subsequently, the fibroblasts will transform into smooth muscle cells to become myofibroblasts capable of contracting and solidifying the bud thus formed. Keratinocytes (endothelial cells) will differentiate to form the protective outer layer of the skin: the stratum corneum. The colonization of the new skin thus reconstituted by specialized cells such as Langerhans cells or melanocytes can begin.
The maturation & remodeling phase
This is the longest phase of healing. It can last from a few weeks to a few months. It begins with the remodeling of the cell matrix with a first inflammatory and proliferative phase that can last 2 months after the formation of the bud. Then, the bud becomes scarce into fibroblasts and a collagen structure appears with a vascularization network that is organized. The mechanical strength of the scar increases and reaches 90% of its final strength around the 6th week. New compounds are then secreted to increase the density of the matrix and its stability.
Age, tension forces, pressure influence the synthesis and organization of collagen molecules. Scars are, however, in any case, less resistant and less elastic than normal skin, partly because of a certain elastin deficiency.
Table of the 4 phases of healing
Treatment of a scar
Most acute wounds (traumatic, superficial burns, surgical...) evolve towards a normal healing process and whose healing time depends on the surface, depth and terrain.
Chronic wounds (bedsores, ulcers, vascular lesions of the diabetic ...) evolve towards a complicated healing process where prevention remains the best treatment. The management and follow-up of (chronic) wounds must be done under medical supervision given the great risks of necrosis.
Several studies have shown that healing is faster in a humid environment than in a dry environment: a 2.5 cm² pig wound under occlusive dressing maintaining a humid area reaches 90% of its healing in 3 days, while healing is only 50% for a wound dried in the open air and even reaches 18% for a wound treated with a stream of warm air.
Exclusion of antiseptics & antibiotics
The first step in treating an acute wound is to exclude any systematic use of antiseptics or local antibiotics.
The interest of using antiseptics on damaged skin is minimal compared to their potential side effects (systemic toxicity, germ selection, allergies, causticity ...). Washing with saline or clean water and soap is more than enough.
As for antibiotics, it should be noted that the bacterial flora, except in excess, is not deleterious, or even participates in the detersion; in the detergent phase, these are essentially Gram-negative bacteria or anaerobes, which will spontaneously decrease with healing, to be replaced by Gram-positive coccis at the budding and epidermization stages. Local antibiotics are therefore generally useless; only silver sulfadiazine continues to be regularly used, especially in Burn Treatment Centers.
Maintaining the moist environment of the injured skin: the dressing
The initial plasma exudation (plasma leakage in case of cut or injury to the skin) constitutes for the fibroblast and the cells of the epidermis an excellent culture medium that should not be disturbed by an overly aggressive attitude (use of certain antiseptics or dressings too aggressive).
Dressings, of great diversity, are the best assets to promote or at least not disturb the healing process, to protect the wound, to control pain, to be permeable to gas, to be well tolerated by the skin, to be easy to change, comfortable, with an acceptable cost/effectiveness benefit.
Dressings are classified into 3 main categories that are:
- Dry dressings (compresses, ready-to-use dressings)
- Wet dressings (the majority of primary dressings)
- And molecular and cellular dressings (transition with dermo-epidermal grafts)
To date, the different dressings existing on the market are divided into the following main families:
- Hydrocolloids used from the detergent phase to the healing phase on exudative wounds
- Hydrogels that have a use comparable to hydrocolloids but more active on necrosis plaques
- Hydrocellular are used in wounds with little or medium exudation from the detergent phase to the healing phase
- Alginates have a high absorption capacity and accelerate the detersion process. They have hemostatic and antibacterial power
- Hydrofibres are close to hydrocolloids and alginates. They can be used from the detergent phase to the healing phase. They are transformed by their hydrophilic quality into a gel in contact with exudates.
- Anthrax dressings especially in chronic smelly or infected wounds
Hydrocolloid dressings
They are all derived from an absorbent polymer, carboxy-methyl-cellulose (CMC). They have significant absorbing capacities. Their interest is based on their simplicity of use, their lifespan of at least 2 days depending on the exudate. Their disadvantage is that they turn in contact with the wound into a smelly gel outside of any infection, which can flow out of the dressing irritating the skin around, worrying the patient ... They therefore sometimes promote maceration, but also hyper budding, reducible by local corticosteroids, and much more rarely contact eczema.
Hydrocellular dressings
Hydrocellular more recently put on the market, avoid the disadvantages of hydrocolloid dressings while absorbing and maintaining a humid environment: they are therefore particularly interesting in partially dug up and moderately exudative wounds.
Hydrogel dressings
Hydrogels consist of 80% water: intended for dry, necrotic wounds, they are actually more humidifying than absorbent. They require a secondary dressing with little absorption, such as a film or a hydrocellular, so that their water goes into necrosis... and not in the compress!
Alginate and hydrofiber dressings
These 2 types of dressings have a high absorption capacity. They come in the form of compresses or strands, which turn into a gel on contact with exudates.
Alginates are extracts of seaweed that are highly absorbent (10 to 15 times their weight) and have hemostatic capacities. They detergent the wound (debris captured by the gel) and control bacterial proliferation by physical trapping; they do not disintegrate into the wound and its removal is non-painful. They are not indicated on non-exudative wounds.
Hydrofibers are dressings based on CMC fiber absorbing 30 times its weight, with an absorption capacity greater than alginates and therefore indicated in exudative wounds, where it turns into a kind of gel.
The use of these 2 types of dressings should not be continued when the flow is reduced because they become very painful.
Charcoal dressings
These are dressings based on a coal knit impregnated with silver ions or not. They limit bacterial overgrowth and odors. They have the disadvantage of being absorbent and they are indicated for fibrinous, infected and smelly wounds (cancerous or diabetic wounds). Not very adherent and moderately absorbent, their use requires a secondary dressing to ensure absorption and maintenance.
HYPERTROPHIC AND CHELOID SCARS
Keloids and hypertrophic scars are intradermal tumors corresponding to an inadequate response of the connective tissue to trauma (burns, acne scars, vaccination, insect bites, wounds ...).
The keloid is characterized by a firm intradermal mass on palpation, which can become itchy and / or hypersensitive and whose epidermis is thinned, smooth and tense. It usually appears 3 to 6 months after the trauma, often extends to the periphery with a "crab paws" appearance and then exceeds the limits of the initial lesion. The initial lesions are erythematous; they evolve to a brownish color before fading. They are preferentially distributed to the neck, earlobes, shoulders, arms and more rarely to the genitals, palms, plants and mucous membranes. There are also spontaneous keloids in the black-skinned patient, mainly.
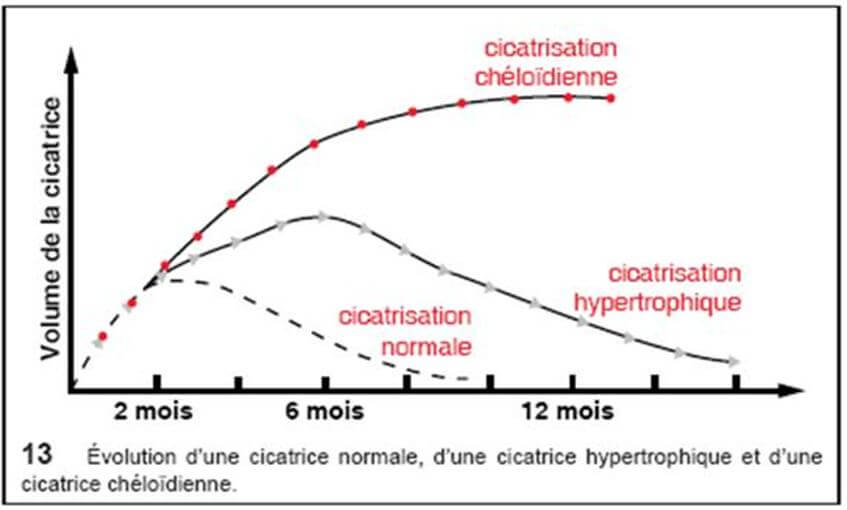
The hypertrophic scar, with parallel edges, does not extend to the periphery; it appears within a month of the trauma and spontaneously progresses to a regression in 12 to 18 months.
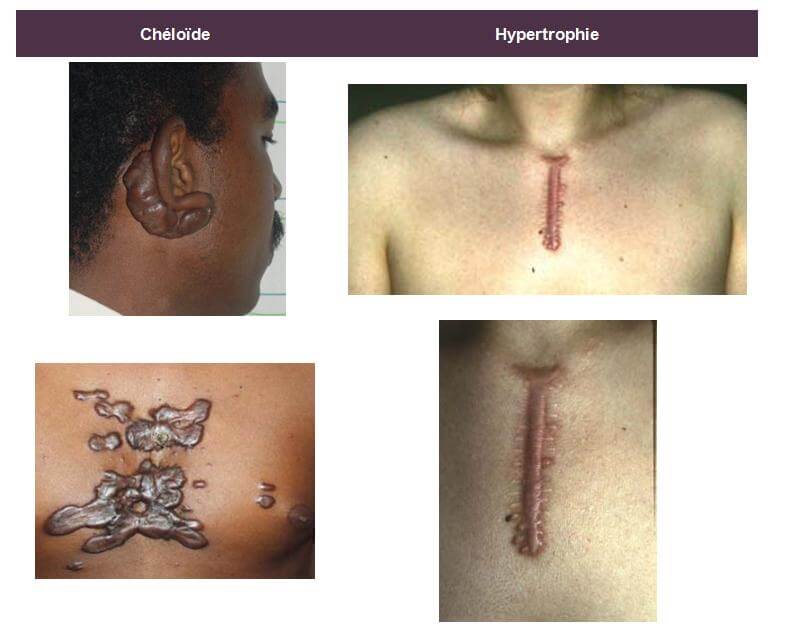
Keloids can affect all ethnic groups but have a higher prevalence for genetically pigmented skin (black African and Asian) with a rate of more than 16% in this type of population.
Keloids
The keloid scar is defined as a fibrous proliferation of dermal origin with root extensions in the form of "crayfish claws" evolving for more than two years. It is a very common lesion in the black race and is a real nightmare for young girls, especially when it sits in exposed areas of the body. Risk factors for keloids are strong pigmentation of the skin (black and dull person), blood type (especially people of group A), age (period of puberty up to 30 years) and localization of physical aggression of the skin (sternum, earlobe, lower part of the face, shoulder stump, neck, pubis), sun exposure of scars.
We have seen above that healing takes place in 4 phases that are: "Hemostasis", "Inflammation", "Budding & Epithelialization" and finally "Maturation and Remodeling". In a keloid, the phase of "Budding & Epithelialization" also called "Proliferative phase" sees its duration explode and goes from a maximum of 21 days on a normal scar to several months on a keloid scar. This hyperactivity of fibroblasts will cause an overproduction of collagen. The absence of collagenases (enzyme responsible for the destruction of collagen) combined with this overproduction of collagen will lead to a swelling in the scar area. The collagen fibers thus synthesized are 20 times more abundant than in normal skin.
Although the formation of a keloid scar raises a lot of controversy, TGF-β seems to be the main factor in this abnormal fibroblast proliferation and collagen production. This abnormal production of TGF-β is associated with an abnormally high concentration of T lymphocytes, macrophages and Langerhans cells from keratinocyte differentiation.
Treatment of keloids
Keloid healing is a pathology specific to humans (no animal develops keloids). This explains the difficulties of understanding the pathophysiology of this anomaly of wound healing for which there is no animal model.
Although some treatments can reduce its severity, there is currently no satisfactory therapeutic solution for this pathology.
Treatment of keloids should be preventive at best, in the frequent case of post-surgical keloids. After the appearance of the keloid, the most effective treatment usually results from the combination of several complementary medical (corticosteroid therapy), surgical and radiation therapeutic procedures.
In addition, the diagnosis of the keloid scar, on which the therapeutic response will depend, is problematic. Its homology of appearance with the hypertrophic scar makes it difficult to make decisions on the therapeutic path in the early phase, where its evolution could be most effectively thwarted.
Corticosteroids
Intra-lesional corticosteroid injection is currently the treatment of choice for keloids. They act on the synthesis of collagen and glycosaminoglycans by decreasing the inflammatory process of the wound, reducing the proliferation of fibroblasts and by a hypoxic effect; they decrease the inhibitors of plasma proteases allowing the degradation of collagen by collagenases; they inhibit the growth of fibroblasts, are responsible for their degeneration and decrease the level of TGF-β. They thus improve the appearance of the lesion without really making it disappear. Triamcinolone acetonide is the reference molecule at a dose of 10 to 40 mg/ml. The injections, painful, are carried out every 3 weeks; 2 to 3 injections are usually required. To increase the effectiveness of corticosteroids, cryotherapy or surgery may be combined.
Cryotherapy
Cryotherapy acts on the keloid by modifying the microcirculation resulting in ischemia, direct cell destruction and tissue necrosis.
The lesion will be cooled for 30 seconds 2 to 3 times per session and this at regular intervals. Highly vascularized keloids present for less than 12 months represent the main indication for this type of treatment.
Radiation therapy
Postoperative interstitial radiotherapy is used mainly in refractory cases. Brachytherapy needles are placed for 24 to 48 hours in the keloid excision scar to prevent its recurrence. Radiotherapy without prior excision is used with much more controversial results. The main side effects are hyperpigmentation, pruritus and erythema. The long-term carcinogenic potential limits its use.
Pressotherapy
Pressotherapy is mainly used in the prevention of hypertrophic scarring in burn victims. External compression should be performed 12 to 24 hours/day for a minimum of 9 months. The pressure on the tissue causes ischemia which decreases tissue metabolism and increases the breakdown of collagen by releasing collagenases during fibroblast apoptosis.
Topical silicone gel
Silicone gel dressings are mainly used for prevention but can also be used in addition to the treatment of hypertrophic scars and keloids.
It is believed that dressings would act more by hydration and occlusion than by the silicone itself.
The laser
CO2 lasers are not recommended on keloids because the risk of recurrence is too high. On the contrary, the pulsed dye laser on white skin can be used for recent, richly vascularized keloids. It acts on erythema, scar thickness and pruritus.
Keloid on black skin, given the high presence of eumelanin (black pigments) is difficult to treat with a laser because of a very high absorption of most wavelengths.
Conclusion on treatments
There is no real consensus for the management of keloids. The first choice remains the intra-lesional injection of corticosteroids. If this treatment is ineffective, intra-marginal surgery with or without injection of corticosteroids or other molecules may be attempted. Radiation therapy should be reserved for the most difficult cases. Pressotherapy and silicone gels will be mainly used as a preventive measure.
In general, the only way to protect against keloids, especially for people at risk, especially the black population, is to avoid any act of aggression of the skin not useful especially at the level of the sternum, the earlobe, the lower part of the face, the stump of the shoulder, the neck and the pubis





